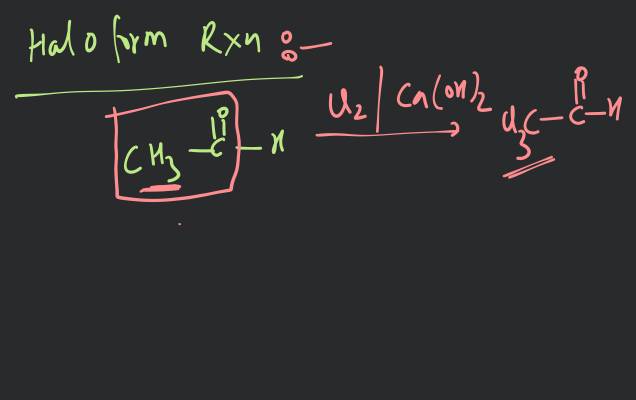Question
Question asked by Filo student

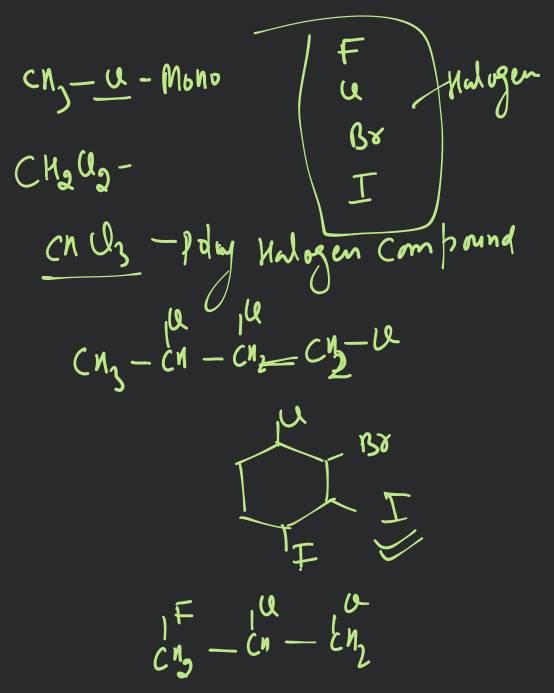
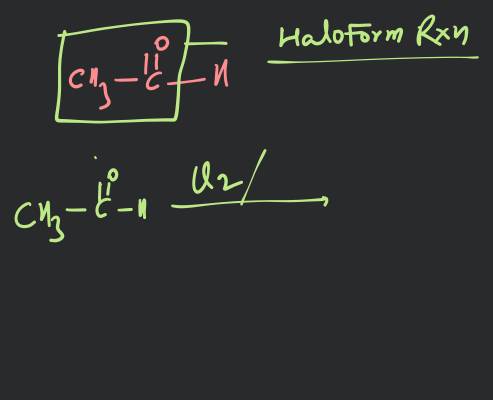
HALOALKANES 10.10. POLYHALOGEN COMPOUNDS Carbon compounds containing more than one halogen atom are called poly halogen colppounds. Many polyhalogen compounds are useful in industry and agriculture. Some of these are described below : 10.10.1. Dichloromethane (Methylene Chloride), Uses. (i) It is widely used as a solvent for paint remover, as a propellant in aerosols and as a process solvent in the manufacture of drugs. (ii) It is also used as metal cleaning and finishing solvent. Physiological effects. Methylene chloride acts on central nervous system (CNS). (i) Exposure to small levels of methylene chloride in air causes slightly impaired hearing and vision. Higher levels of methylene chloride in air causes dizziness, nasea, tingling and numbness in the fingers and toes. (ii) Direct contact of methylene chloride with skin causes intense burming and mild redness of skin. Direct contact with the eyes can bum cornea. The comea of animals was damaged when exposed to vapours of methylene chloride. 10.10.2. Trichloromethane (Chloroform), * Preparation. 1. By distilling ethanol (ethyl alcohol) or propanone (acetone) with a suspension of bleaching powder in water (Labonatory method) : The reaction in case of ethyl alcohol is supposed to take place as under : (i) Bleaching powder supplies both chlorine and calcium hydroxide. (ii) Chlorine acts both as an oxidising as well as a chlorinating agent. Oxidation : Chlorination : or Chloral (iii) Chloral is then hydrolysed by calcium hydroxide to give chloroform. In case of acetone, the reactions are : (i) (ii) Propanone (Aceone (iii) Acetone is preferred to ethyl alcohol, as the yield is better.
Found 4 tutors discussing this question
Discuss this question LIVE
13 mins ago
Filo tutor solutions (3)
Learn from their 1-to-1 discussion with Filo tutors.
11 mins
Uploaded on: 4/21/2023
Was this solution helpful?
99
Share
Report
15 mins
Uploaded on: 4/21/2023
Was this solution helpful?
81
Share
Report

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Practice more questions on Alkyl Halide
Question 2
Easy
Views: 6,494
Question 3
Medium
Views: 5,693
(R): In presence of acts as a base and produces ions.
Students who ask this question also asked
Question 2
Views: 5,210
Question 3
Views: 5,160
Question 4
Views: 5,904


Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text |
HALOALKANES
10.10. POLYHALOGEN COMPOUNDS
Carbon compounds containing more than one halogen atom are called poly halogen colppounds. Many polyhalogen compounds are useful in industry and agriculture. Some of these are described below : 10.10.1. Dichloromethane (Methylene Chloride),
Uses. (i) It is widely used as a solvent for paint remover, as a propellant in aerosols and as a process solvent in the manufacture of drugs.
(ii) It is also used as metal cleaning and finishing solvent.
Physiological effects. Methylene chloride acts on central nervous system (CNS).
(i) Exposure to small levels of methylene chloride in air causes slightly impaired hearing and vision. Higher levels of methylene chloride in air causes dizziness, nasea, tingling and numbness in the fingers and toes.
(ii) Direct contact of methylene chloride with skin causes intense burming and mild redness of skin. Direct contact with the eyes can bum cornea. The comea of animals was damaged when exposed to vapours of methylene chloride.
10.10.2. Trichloromethane (Chloroform), *
Preparation.
1. By distilling ethanol (ethyl alcohol) or propanone (acetone) with a suspension of bleaching powder in water (Labonatory method) :
The reaction in case of ethyl alcohol is supposed to take place as under :
(i) Bleaching powder supplies both chlorine and calcium hydroxide.
(ii) Chlorine acts both as an oxidising as well as a chlorinating agent.
Oxidation :
Chlorination : or Chloral
(iii) Chloral is then hydrolysed by calcium hydroxide to give chloroform.
In case of acetone, the reactions are :
(i)
(ii) Propanone (Aceone
(iii) Acetone is preferred to ethyl alcohol, as the yield is better. |
| Updated On | Apr 21, 2023 |
| Topic | Alkyl Halide |
| Subject | Chemistry |
| Class | Class 12 |
| Answer Type | Video solution: 3 |
| Upvotes | 240 |
| Avg. Video Duration | 11 min |