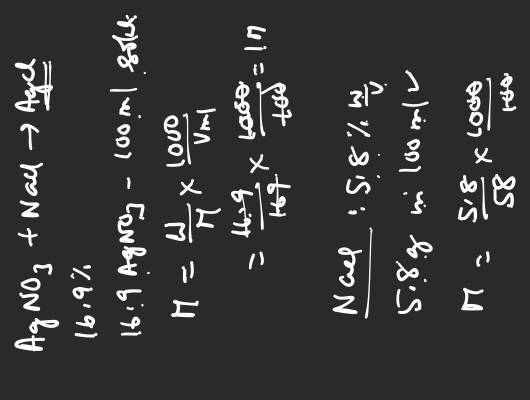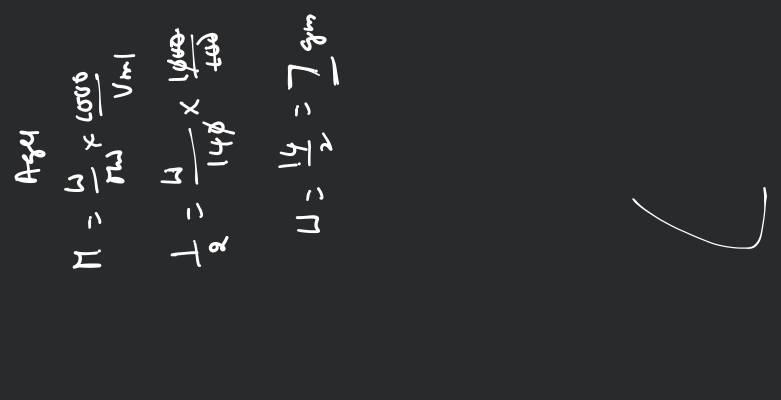Question
Medium
Solving time: 3 mins
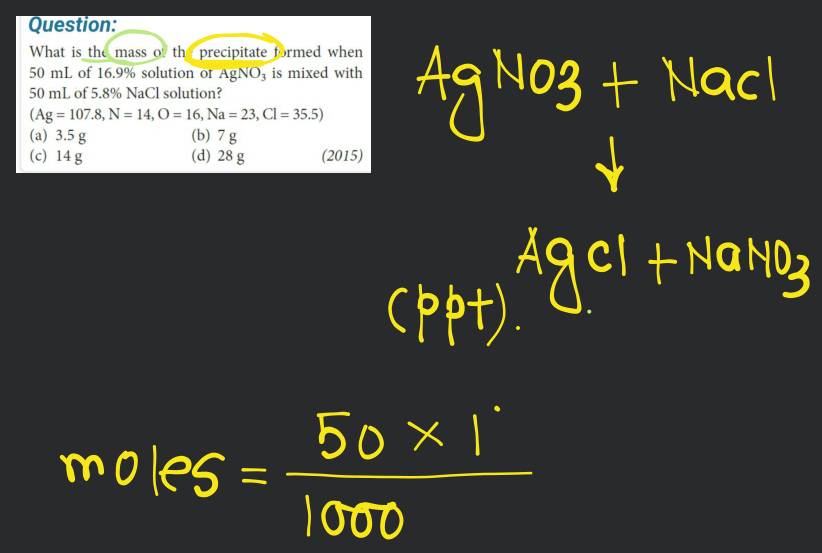
What is the mass of the precipitate formed when 50 mL of 16.9% solution of is mixed with 50 mL of 5.8% NaCl solution?
Found 7 tutors discussing this question
Discuss this question LIVE
13 mins ago
 Text solution
Text solution Verified
Verified
16.9 g is present in 100 mL solution.
∴ 8.45 g is present in 50 mL solution
5.8 g NaCl is present in 100 mL solution
∴ 2.9 g NaCl is present in 50 mL solution

Mass of AgCl precipitated
= 0.049 × 143.5 g
= 7 g AgCl
∴ 8.45 g is present in 50 mL solution
5.8 g NaCl is present in 100 mL solution
∴ 2.9 g NaCl is present in 50 mL solution
Mass of AgCl precipitated
= 0.049 × 143.5 g
= 7 g AgCl
Was this solution helpful?
31
Share
Report
Filo tutor solutions (28)
Learn from their 1-to-1 discussion with Filo tutors.
7 mins
Uploaded on: 1/21/2023
Was this solution helpful?
146
Share
Report
19 mins
Uploaded on: 4/20/2023
Was this solution helpful?
52
Share
Report

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Questions from NEET 2015 - PYQs
Question 1
Easy
Views: 6,115
Question 2
Medium
Views: 5,571
Question 3
Easy
Views: 5,906
Question 4
Medium
Views: 6,508
Practice questions from Linear Algebra: A Modern Introduction
Question 1
Medium
Views: 5,642
(i) Calculate the mass of ammonia produced if g dinitrogen reacts with g of dihydrogen.
(ii) Will any of the two reactants remain unreacted?
(iii) If yes, which one and what would be its mass?
(ii) Will any of the two reactants remain unreacted?
(iii) If yes, which one and what would be its mass?
Question 2
Easy
Views: 6,285
Question 3
Easy
Views: 6,175
Question 4
Easy
Views: 6,074
Practice questions from Some Basic Concepts of Chemistry in the same exam
Question 1
Easy
Views: 6,571
(At. wt. )
Question 2
Medium
Views: 5,739
Practice more questions from Some Basic Concepts of Chemistry
Question 1
Medium
Views: 5,101
Question 2
Medium
Views: 6,093
Practice questions on similar concepts asked by Filo students
Question 1
Views: 5,241
Question 2
Views: 6,028
Question 3
Views: 5,537
Question 4
Views: 5,642


Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | What is the mass of the precipitate formed when 50 mL of 16.9% solution of is mixed with 50 mL of 5.8% NaCl solution? |
| Updated On | May 2, 2023 |
| Topic | Some Basic Concepts of Chemistry |
| Subject | Chemistry |
| Class | Class 11 |
| Answer Type | Text solution:1 Video solution: 28 |
| Upvotes | 2900 |
| Avg. Video Duration | 8 min |