Question
Hard
Solving time: 5 mins
Q.181. Consider the possible vibration modes in the following linear molecules:
(a) CO2 (O — C—O); (b) C2H2 (H—C —C—H).
Found 8 tutors discussing this question
Discuss this question LIVE
14 mins ago
 Text solution
Text solution Verified
Verified
(a) CO2 (O - C - O)
The molecule has 9 degrees of freedom 3 for each atom. This means that it can have up to nine frequencies. 3 degrees of freedom correspond to rigid translation, the frequency associated with this is zero as the potential energy of the system can not change under rigid translation. The P.E. will not change under rotations about axes passing through the C-atom and perpendicular to the O - C - O line. Thus there can be at most four non-zero frequencies. We must look for modes different from the above.
One mode is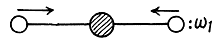
Another mode is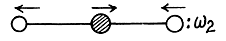
These are the only collinear modes.
A third mode is doubly degenerate:
(vibration in and to the plane of paper).
(b) C2H2 ( H - C - C - H )
There are 4 x 3 - 3 - 2 = 7 different vibrations. There are three collinear modes.
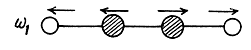

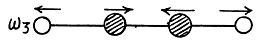
Two other doubly degenerate frequencies are

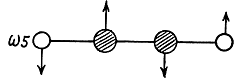
together with their counterparts in the plane to the paper.
The molecule has 9 degrees of freedom 3 for each atom. This means that it can have up to nine frequencies. 3 degrees of freedom correspond to rigid translation, the frequency associated with this is zero as the potential energy of the system can not change under rigid translation. The P.E. will not change under rotations about axes passing through the C-atom and perpendicular to the O - C - O line. Thus there can be at most four non-zero frequencies. We must look for modes different from the above.
One mode is
Another mode is
These are the only collinear modes.
A third mode is doubly degenerate:
(vibration in and to the plane of paper).
(b) C2H2 ( H - C - C - H )
There are 4 x 3 - 3 - 2 = 7 different vibrations. There are three collinear modes.
Two other doubly degenerate frequencies are
together with their counterparts in the plane to the paper.
Was this solution helpful?
35
Share
Report

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Practice questions from Problems in General Physics (IE Irodov)
Question 1
Hard
Views: 5,312
Question 2
Hard
Views: 5,149
Question 3
Hard
Views: 5,819
Question 4
Hard
Views: 5,283
Practice more questions from Nuclei
Question 1
Easy
Views: 6,007
Reason: In -decay the mass number decreases by 4 and atomic number decreases by 2 . In -decay the mass number remains unchanged, but atomic number increases by 1 only.
Question 2
Easy
Views: 5,750
Practice questions on similar concepts asked by Filo students
Question 1
Views: 5,261
Question 2
Views: 5,677
(R) : The charge of proton is 1837 times more than the charge of electron.a. Both 'A' and 'R' are true and 'R' is the correct explanation of 'A'. b. Both 'A' and 'R' are true and 'R' is not the correct explanation of 'A' c. 'A' is true and 'R' is false d. 'A' is false and 'R' is false
Question 4
Views: 5,858


Stuck on the question or explanation?
Connect with our Physics tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | Q.181. Consider the possible vibration modes in the following linear molecules: (a) CO2 (O — C—O); (b) C2H2 (H—C —C—H). |
| Topic | Nuclei |
| Subject | Physics |
| Class | Class 12 |
| Answer Type | Text solution:1 |
| Upvotes | 35 |



