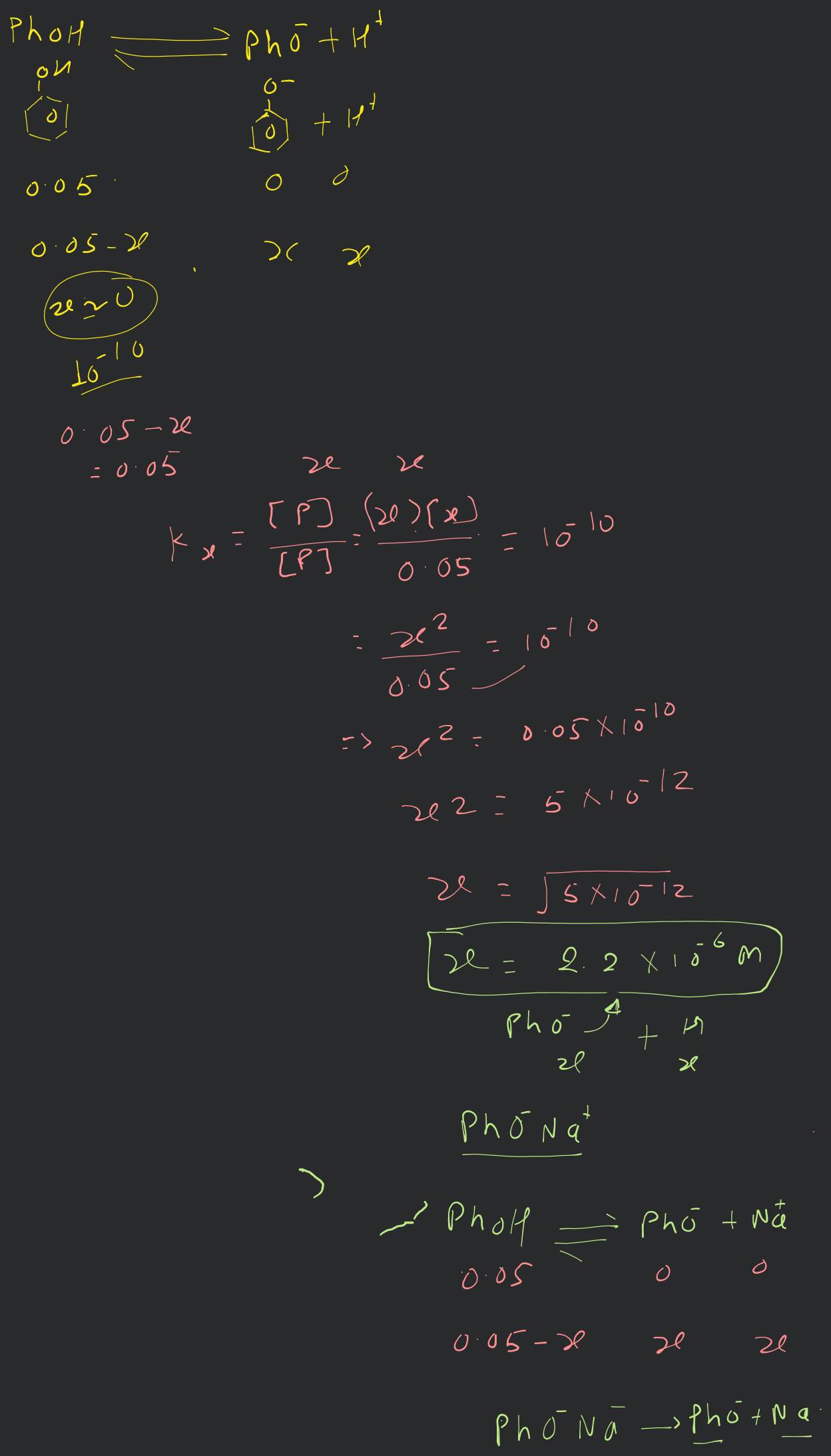Question
Medium
Solving time: 3 mins
The ionization constant of phenol is . What is the concentration of phenolate ion in solution of phenol? What will be its degree of ionization if the solution is also in sodium phenolate?
Found 6 tutors discussing this question
Discuss this question LIVE
9 mins ago
 Text solution
Text solution Verified
Verified
The balanced chemical equation for the ionization of phenol is:
.
The initial concentrations of phenol, phenoxide ion and hydronium ion are 0.05, 0 and 0 respectively.
Corresponding equilibrium concentrations are x and x respectively.
x is very small (as the ionization constant is very small) . Hence, x can be ignored in the denominator.
Let be the degree of dissociation of phenol in presence of 0.01 M phenoxide.
The dissociation of phenoxide is as represented:
Also,
M (as the degree of dissociation is very small.)
M
.
The initial concentrations of phenol, phenoxide ion and hydronium ion are 0.05, 0 and 0 respectively.
Corresponding equilibrium concentrations are x and x respectively.
x is very small (as the ionization constant is very small) . Hence, x can be ignored in the denominator.
Let be the degree of dissociation of phenol in presence of 0.01 M phenoxide.
The dissociation of phenoxide is as represented:
Also,
M (as the degree of dissociation is very small.)
M
Was this solution helpful?
Share
Report
Filo tutor solutions (3)
Learn from their 1-to-1 discussion with Filo tutors.
6 mins
Uploaded on: 8/24/2023
Was this solution helpful?
143
1
Share
Report
9 mins
Uploaded on: 8/24/2023
Was this solution helpful?
94
Share
Report

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Practice more questions from Chemistry Part-I (NCERT)
Q1
The ionization constant of and at 298K are and respectively. Calculate the ioniation constants of the corresponding conjugate base.
Q2
The ionization constant of phenol is . What is the concentration of phenolate ion in solution of phenol? What will be its degree of ionization if the solution is also in sodium phenolate?
Q3
The first ionization constant of is . Calculate the concentration of ion in its solution. How will this concentration be affected if the solution is in also? If the second dissociation constant of is , calculate the concentration of under both conditions.
View allPractice questions from Chemistry Part-I (NCERT)
Question 1
Medium
Views: 6,161
Question 2
Easy
Views: 5,587
a) .
b) the equilibrium constant for the formation of from NO and at 298 K.
where kJ/mol kJ/mol kJ/mol.
Question 3
Easy
Views: 5,875
the value of the equilibrium constant, is at . Calculate the for the reaction at this temperature?
Question 4
Medium
Views: 6,275
Practice more questions from Equilibrium
Question 1
Medium
Views: 5,565
at .
The standard enthalpy of formation of is kJmol and the standard entropies of , and are , and JK mol, respectively.
Question 2
Medium
Views: 5,991
Question 4
Hard
Views: 5,861
Practice questions on similar concepts asked by Filo students
Question 1
Views: 5,514
Question 2
Views: 5,703
Question 3
Views: 5,961
Question 4
Views: 5,664


Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | The ionization constant of phenol is . What is the concentration of phenolate ion in solution of phenol? What will be its degree of ionization if the solution is also in sodium phenolate? |
| Updated On | Aug 24, 2023 |
| Topic | Equilibrium |
| Subject | Chemistry |
| Class | Class 11 |
| Answer Type | Text solution:1 Video solution: 3 |
| Upvotes | 311 |
| Avg. Video Duration | 7 min |